
Geoffrey E. Lloyd
and Noel W. Thomas
(School of Earth Sciences, University
of Leeds):
VORONOI POLYHEDRAL MODELLING: FROM CRYSTAL CHEMISTRY TO PETROPHYSICS
Use of Voronoi polyhedra to model phase transformations and reactions is based on:
1. variation in ionic polyhedral volumes and ratios;
2. interpretation of shared polyhedral faces;
3. polyhedral geometry.
The faces of Voronoi polyhedra correspond to pairwise interactions (i.e. ‘bonds’), from which face interaction indices can be defined, leading to the construction of phase structure maps. Alternatively, shared polyhedral vertices correspond to structural voids which are natural sampling points to probe clustering, introducing the concept of structural fingerprinting of phases.
As the characteristics of Voronoi polyhedra change with (P, T, x), it is possible to simulate geometrically the effect of one variable on the others using experimentally constrained data. Such changes can be used to model the behaviour of minerals in terms of their stability and/or tendency for transformation and/or reaction.

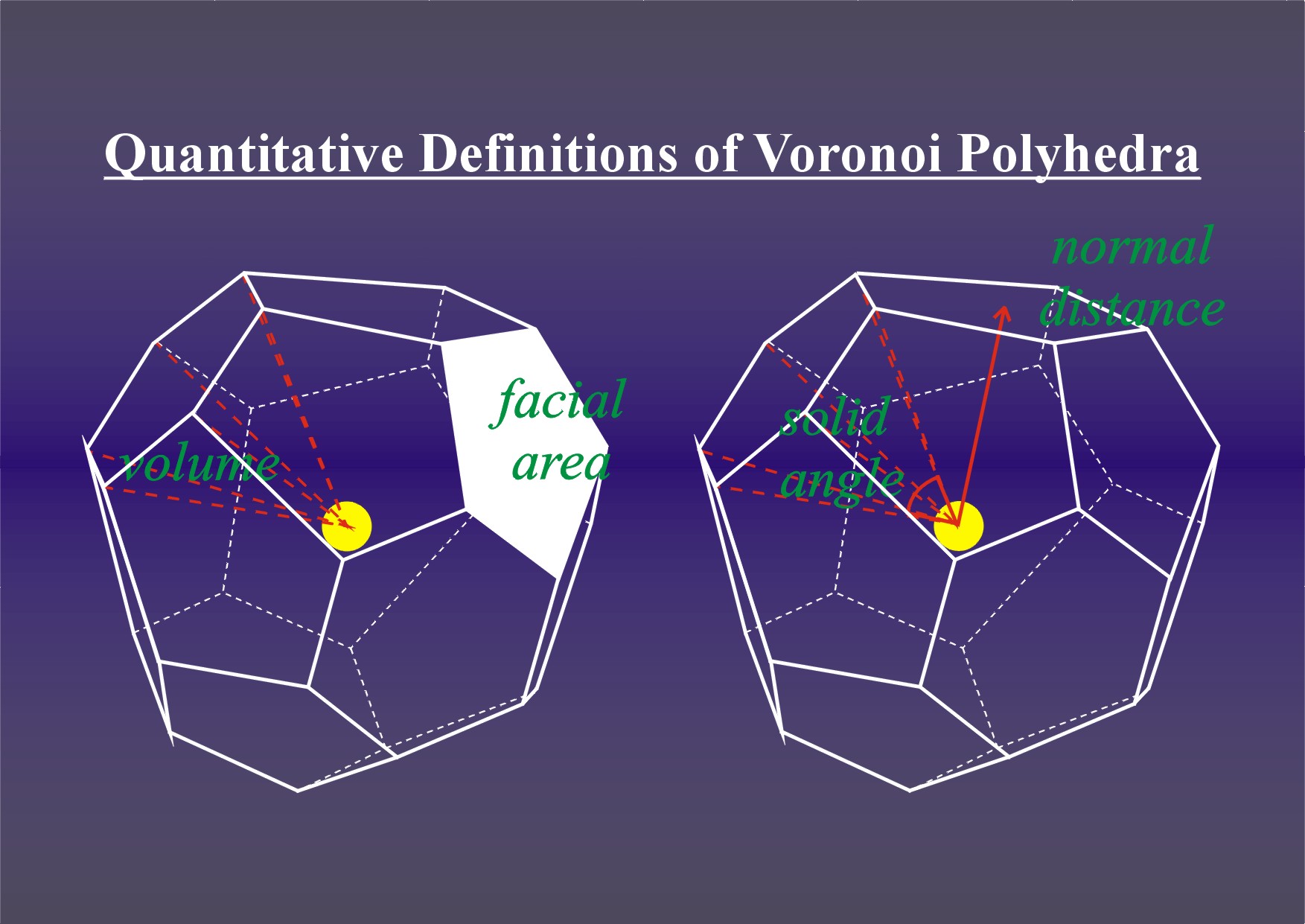
Figure 1. Voronoi polyhedra constructed about individual ions, showing the various paramaters available to quantify crystal chemical modelling of mineral phase transformations and metamorphic reactions.
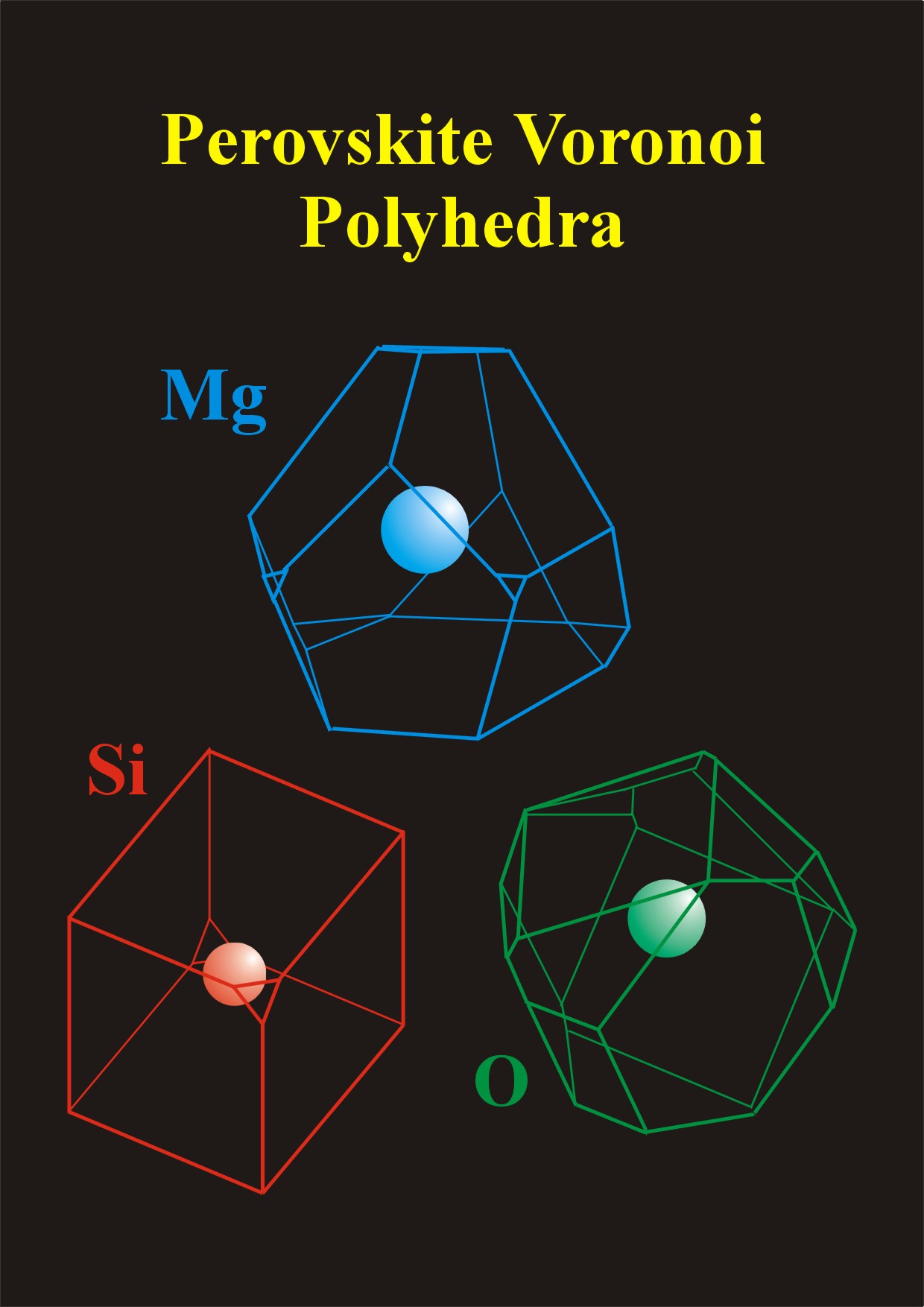
Figure 2. Examples of Voronoi polyhedra for magnesium silicate perovskite. The simple polyhedra (i.e. few faces) associated with Si ion suggests that this is structurally stable compared with the Mg and, especially, the O ions, which have more complex polyhedral shapes and volumes.
Return to Workshops
Last updated: May 19, 1999